Analytical Method Transfer Protocol Template

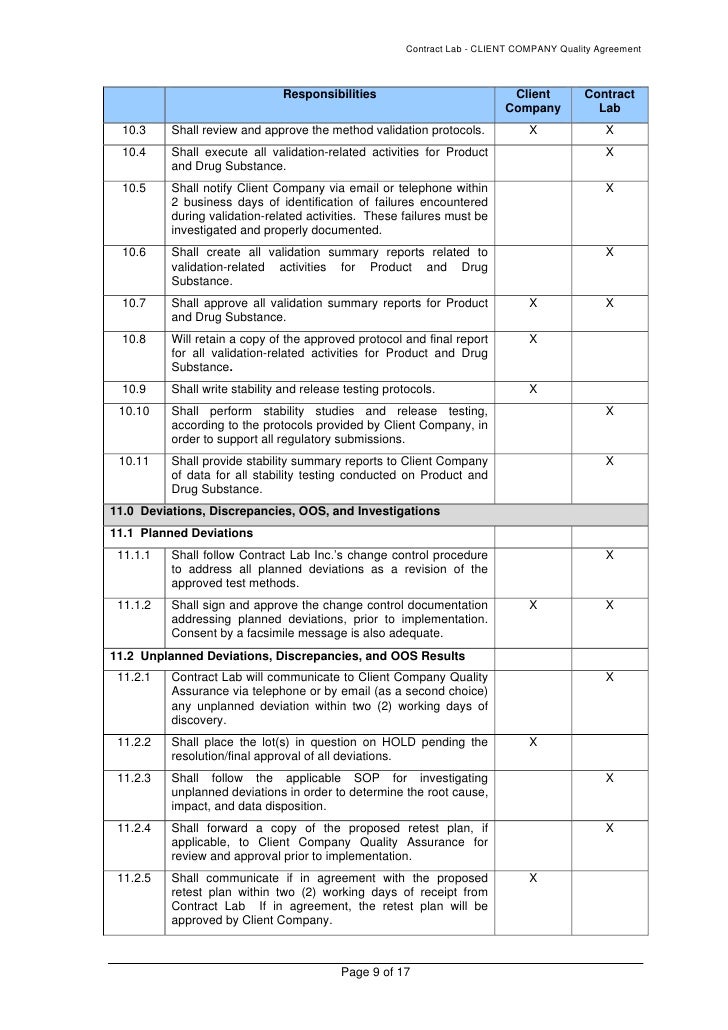
The receiving laboratory shall verify that all equipmentsystems required to perform the method testing is available the receiving laboratory shall verify that all equipment is qualified and properly calibrated laboratory equipmentsystems are in compliance with all applicable regulations and user specifications.
Analytical method transfer protocol template. Method transfer at a glance. 541 upon completion of analytical method transfer data shall be compiled. What are we trying to achieve with a method transfer. When undertaking analytical method transfer it is important to base the approach on the stage of development.
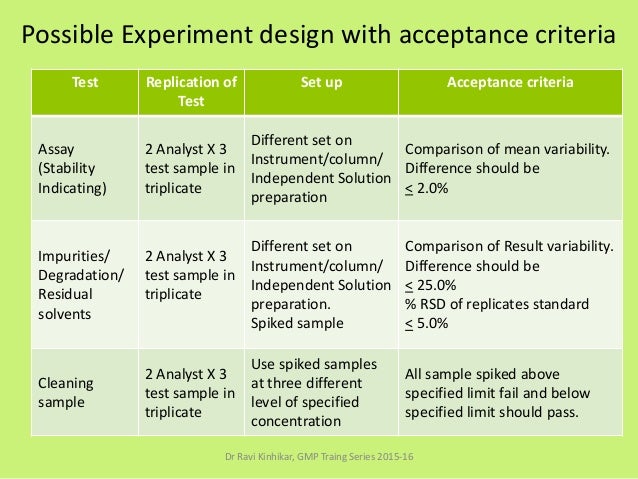
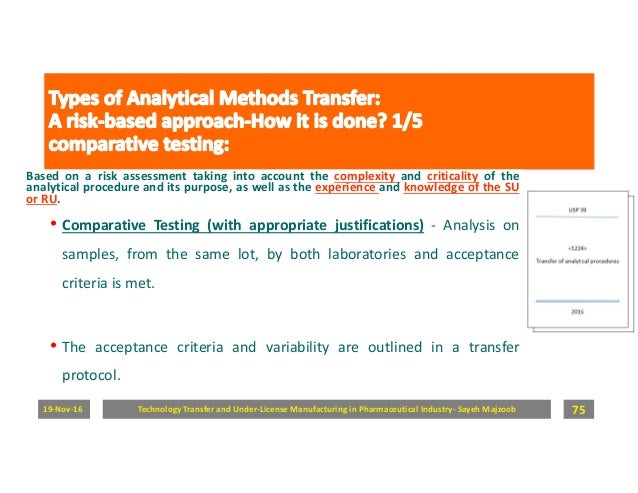
3 steps to a successful analytical method transfer of your solid oral dosage form john frankonislaboratory director ropack pharma solutions the analytical testing of intermediate and finished drug products is a critical part of the drug development process as it is essential to ensuring the safety and efficacy of the final product. Analytical method transfer shall be considered successful if results obtained by the analysis performed during method transfer lies within the predetermined acceptance criteria as per the approved protocol. Transfer package was detailed method was standard nothing out of the ordinary ru passed the transfer ru failed first batches of api ac set to 10 ru results were 08 for all results transfer deemed success and finalized assay results historically averaged 985 bias in the method was traced to weighing techniques. Prepare a protocol the first step in method validation is to prepare a proto col preferably written with the instructions in a clear step by step format and approved prior to their initiation.
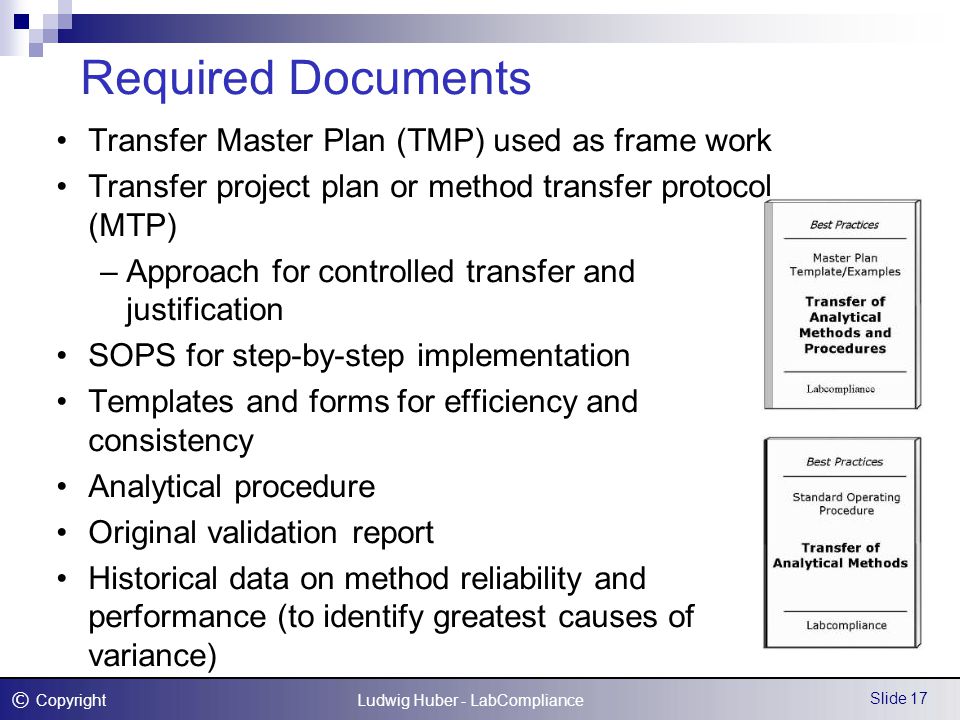
54 analytical method transfer shall be carried out by using approved protocol given by rd or contract giver. Analytical method transfer this section should outline the method validation and transfer strategies to be applied. Report shall be prepared and sent to rd or contract giver for review. Qualify a new laboratory.
And ability to perform the transferred analytical procedure as intended usp1224 ability to perform the method should. This approach is discussed in this paper. Success criteria for analytical method transfer. The receiving site will use these standard monographs to.
An analytical method is transferred from a sending unit su to the receiving unit ru. To perform an analytical method qualification ensures the receiving lab has the. Analytical method transfer checklist. 53 technology transfer documents shall be received from rd or contract giver.
The analytical methods used for starting materials originate from standard insert relevant compendia and codex references bp usp fcc etc.