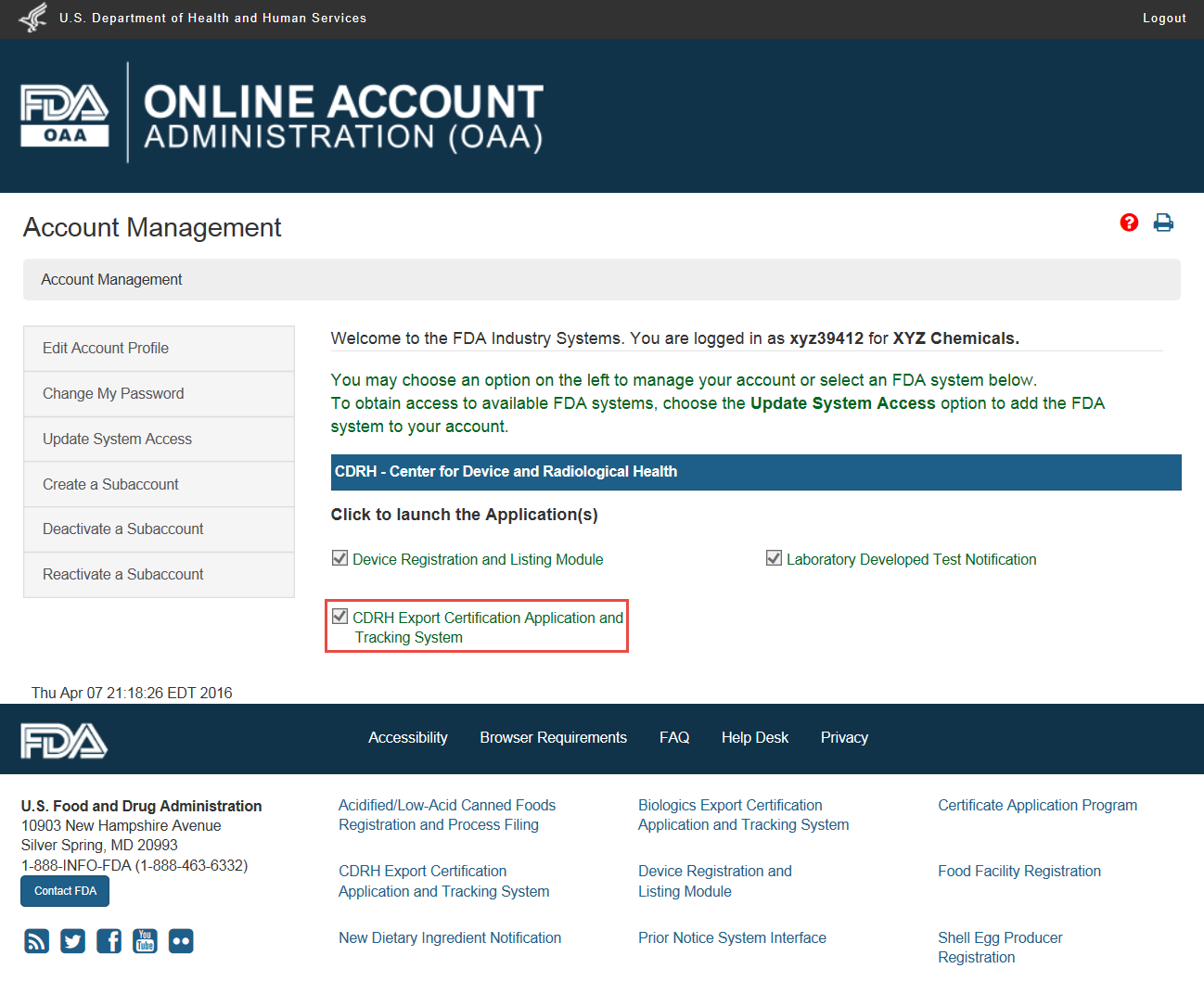
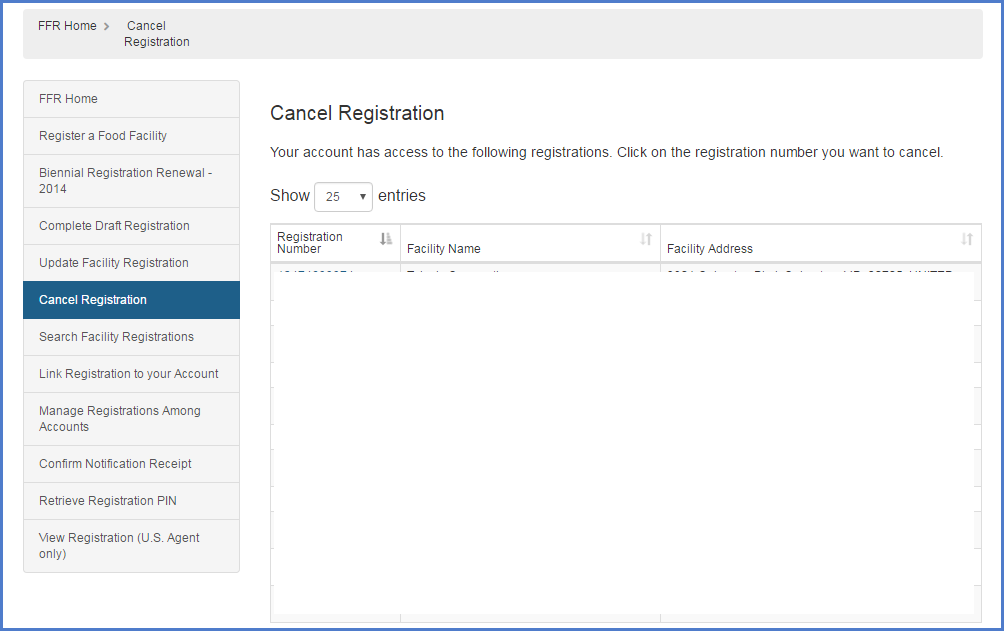
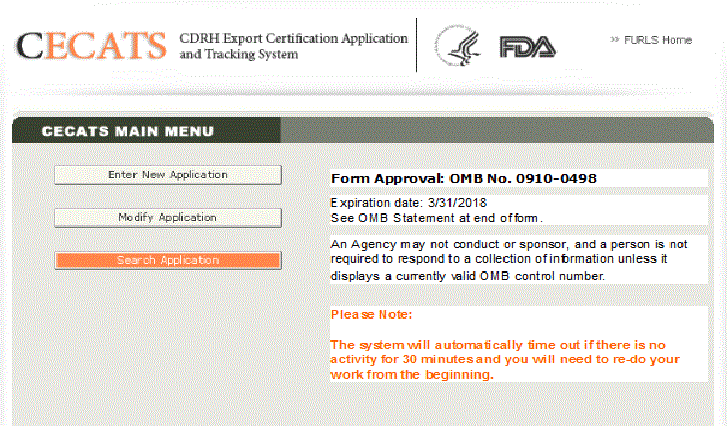
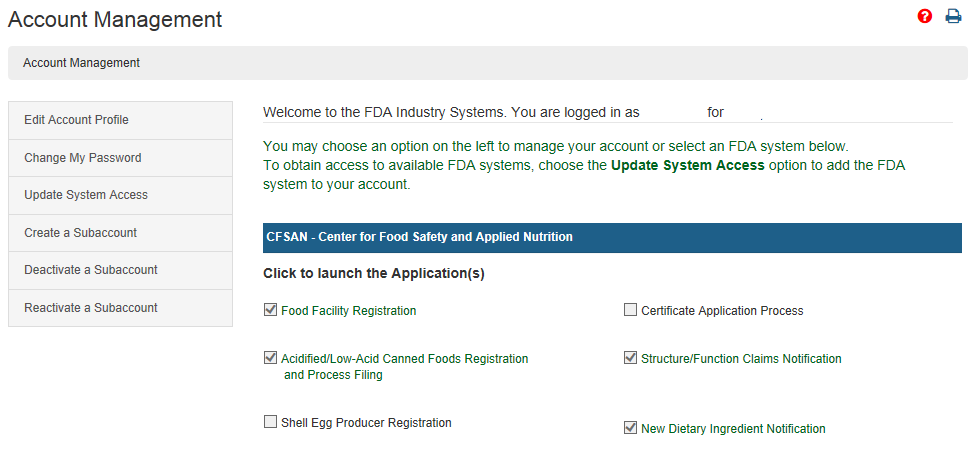
Fda Certification Lookup
Any representation of fda registration number on product label or labeling which implies fda certification or fda approval of a facility or product is misleading and may cause misbranding of the product.
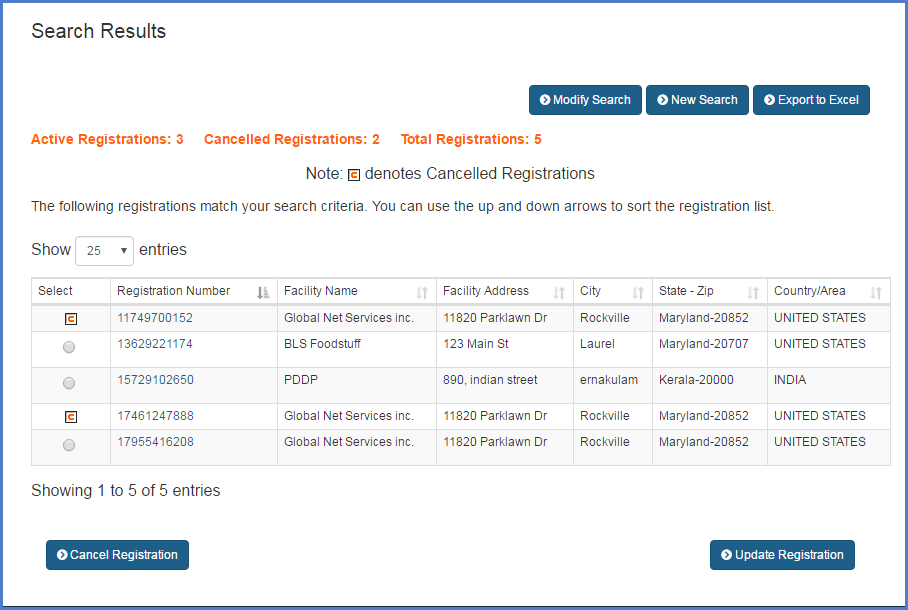
Fda certification lookup. Releasable establishment registration and listing information under the freedom of information act is available by searching the establishment registration and listing database. You may use this application to review human cell and tissue registration information for registered inactive and pre registered firms. These inspection classifications may or may not represent the final agency determination of compliance for these firms. A 510k is a premarket submission made to fda to demonstrate that the device to be marketed is at least as safe and effective that is substantially equivalent to a legally marketed device 21 cfr 80792a3 that is not subject to premarket approval.
Search fda issued warning letters by keyword or use our advanced search functionality to search by company date issued issuing office subject or whether a response letter or closeout letter is. Department of justice drug enforcement administration diversion control division maintains registrant data and is considered the primary source of information on dea registrants. Welcome to the hcters public query application. Inspection classifications listed in this report reflect the compliance status of firms when the report was generated.
Fda registration or fda registration number does not denote fda certification or approval of your facility or products. Please keep in mind that provision of the mammography facility database of mqsa certified facilities does not mean that fda or any other organization recommends one certified facility over another. For human drug biologic animal drug and device export certificates issued under section 801e4 of the act. The website httpswwwdeadiversionusdojgov is the official location for real time online verification.
The agency may charge a fee of up to 175 if fda issues a certificate within 20. The database is.