Fda Cgmp Certification

Find education and resources related to fdas regulatory product quality and safety responsibilities.
Fda cgmp certification. Current good manufacturing practice cgmp regulations. Cgmp refers to the current good manufacturing practice regulations enforced by the fda. Fdas cgmp requirements for drugs are the requirements for the methods to be used in and the facilities or controls to be used for the manufacture processing packing or holding of a drug. Center for food safety and applied nutrition.
Food cgmp modernization a focus on food safety november 2 2005. Food cgmp modernization working group. About cgmp certification the cgmp certification is needed by manufacturing companies to manufacture and sell food and drug related products. Comments regarding this document may be submitted at any time.
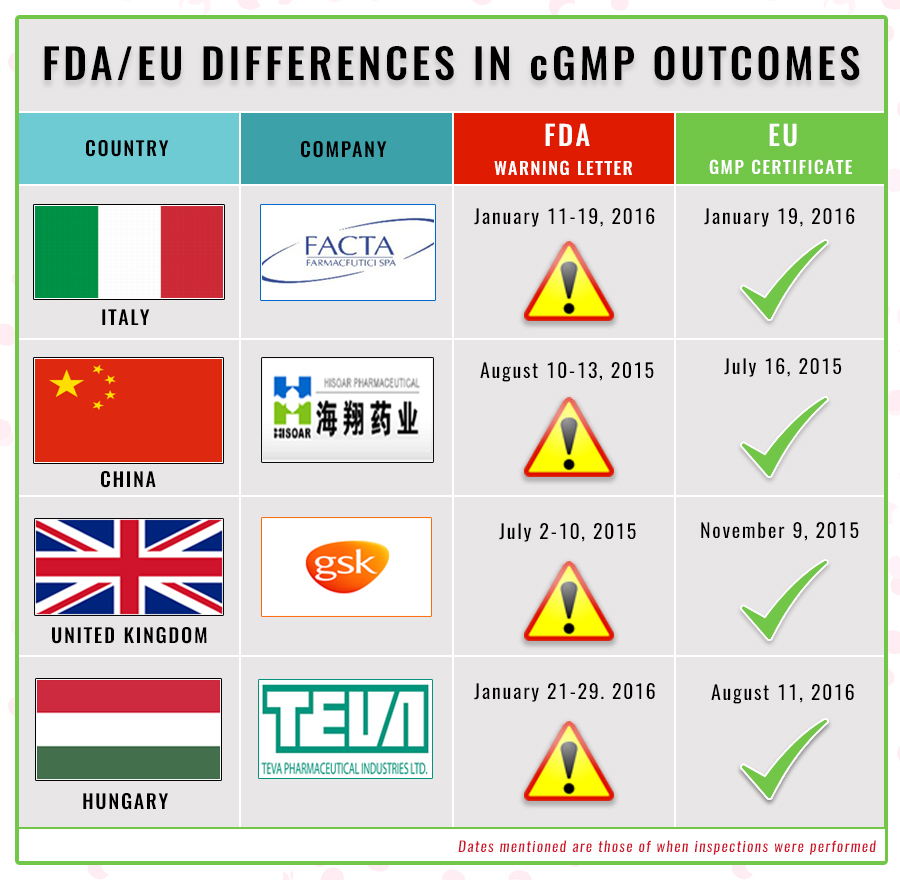
Fda is fulfilling its commitment under the generic drug user fee amendments of 2017 gdufa ii for issuing letters called current good manufacturing practice cgmp declarations to foreign regulators that convey cgmp compliance status of establishments fda has inspected. Cgmps provide for systems that assure proper design monitoring and control of manufacturing processes and. Submit comments to dockets management branch hfa 305 food and drug administration 5630 fishers lane room 1061 rockville md 20852. Fda ensures the quality of drug products by carefully monitoring drug manufacturers compliance with its current good manufacturing practice cgmp regulations.
As part of this commitment. The regulations cgmps are listed inthe cfr code offederal regulationspart 210 and 211 part 210 definitions part 211 basicinstructions part 11 electronicdata. Good manufacturing practices gmps for the 21 st century food processing august 9 2004. Cfpie has developed a good manufacturing practices training and certification program to meet the educational needs of those responsible for compliance with this complex regulation.
The cgmp regulations for drugs contain minimum requirements for the methods facilities and controls used in manufacturing. Modernization of food good manufacturing practice regulations. Fda learning portal for students academia and industry. The regulations cgmp stands for currentgood manufacturingpractices always improving andchanging that is why theyare called current regulations are aminimum that must be met 4.
The gmp or good manufacturing practices are the guidelines that decide whether or not a company is allowed to sell their product to the general public.