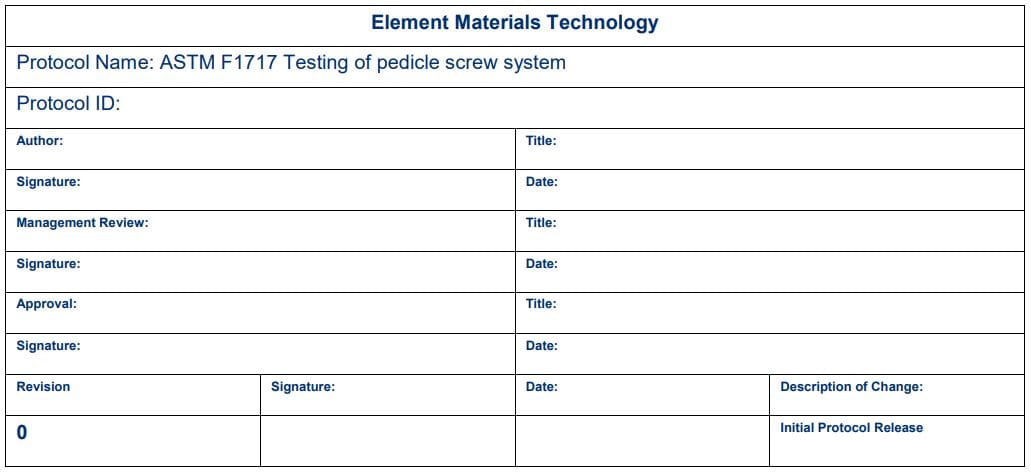
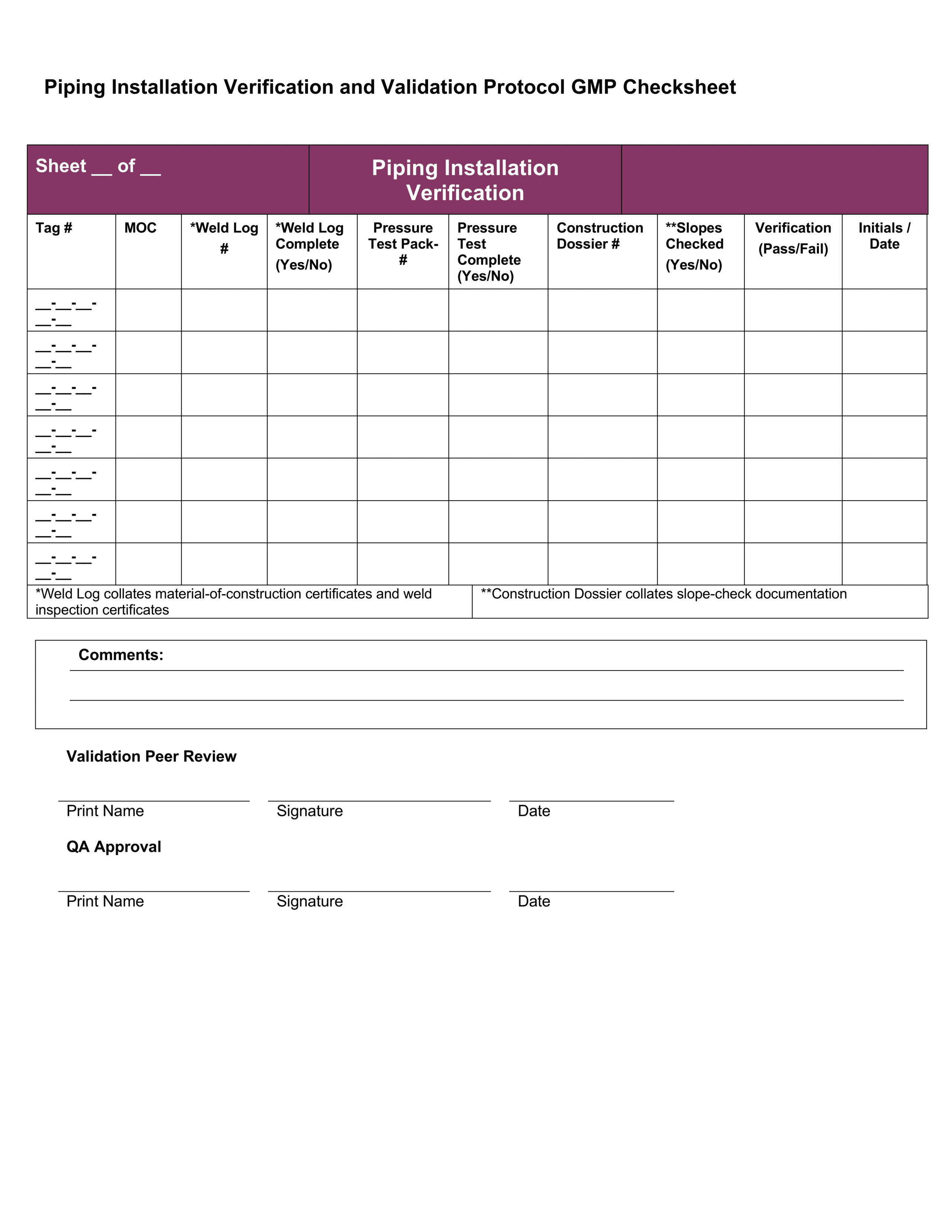
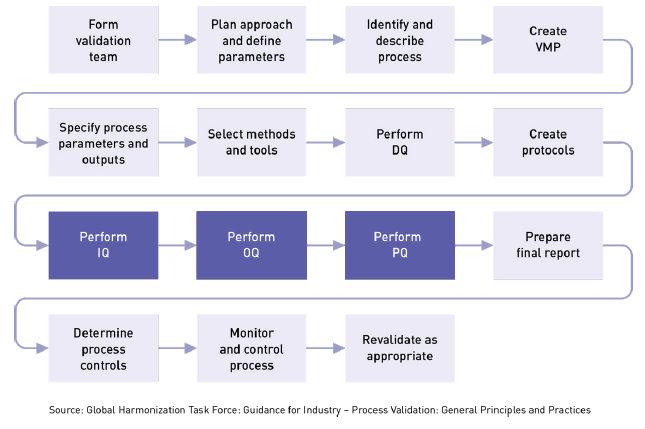
Medical Device Test Protocol Template

Examples of tests which should have plans or protocol written for them include medical non medical field lab or production studies design of experiments doe problem solving reliability or software.
Medical device test protocol template. In these cases the device sponsor may need to develop their own internal test procedure. Identifying the correct fda guidance documents and standards. The test protocol and report must be included in the 510k submission. For the system name.
0 53000 validation verification and testing plan template and checklist rev. For the medical device industry it is critical to identify the worst case configurations for testing. 1 41202 conversion to word 2000 format validation verification and testing plan authorization memorandum i have carefully assessed the validation verification and testing plan. To company given differences in test types strategy scope and industry.
Custom test procedures the proposed vv service approach may provide medical devices manufacturer with custom test procedures that integrate the expertise of both parties about the product testing. Today expect medical device templates validation to perform to a high level. This protocol template has been designed primarily for clinical device trials which are subject to the medicines for human use clinical trials regulations 2004 and amendment 2006. Company axialtorsional elf protocol rev.
When your medical device product development project gets to design verification and design validation do you feel like its almost there. It will meet the validation activity requirements of the partners organization including the documentation of input. It is helpful to include this in the protocol along with any finite element analysis results because this is often the only insight the testing laboratory or future team members have into why this specific device size was selected. At the same time the fda medical device templates business has become highly regulated.
Protocol medical device testing inc. Or is it more like being stuck in the middle. A medical device firm once submitted the only prototype of its electronic system to a local test house for vibration and impact testing. Importance of test planstest protocol with a template.
Meaning finally you can see the goal of market release coming soon. 00 protocol rev 00 page 1 of 7 in vitro accelerated fatigue testing protocol this is a test plan for acclerated torsional fatigue testing of company devicesthe duration of this test simulates 10 years of implantation life. Medical device testing requirements for 510k submissions.