Sodium Bohr Diagram
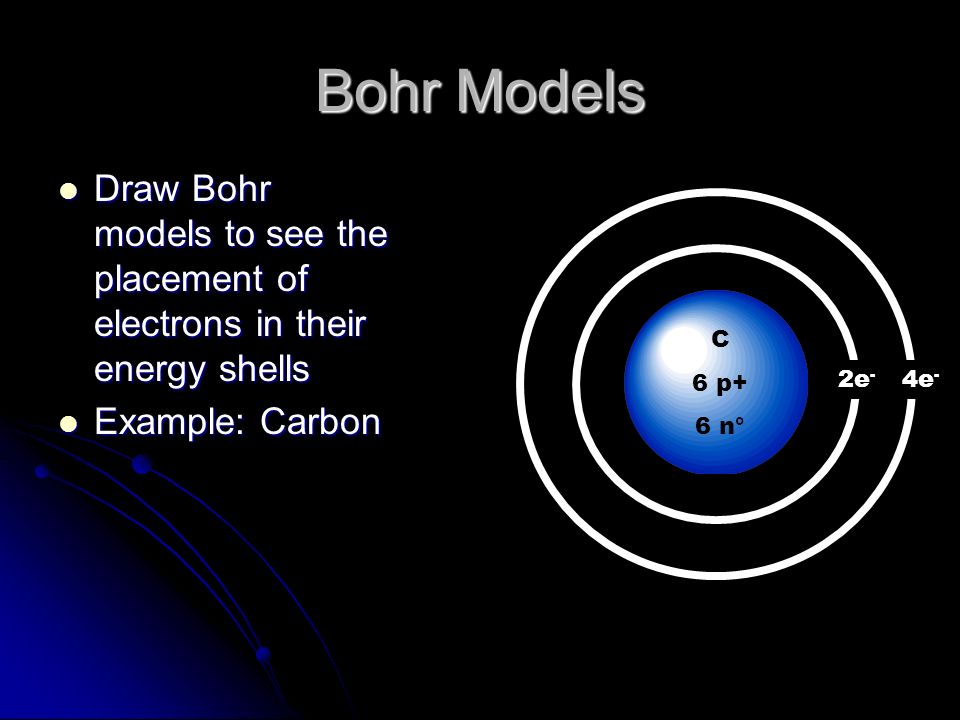
What does bohr diagram of sodium and chlorine to form sodium chloride look like.
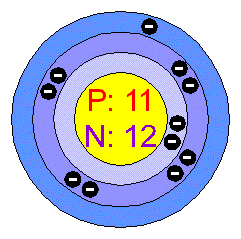
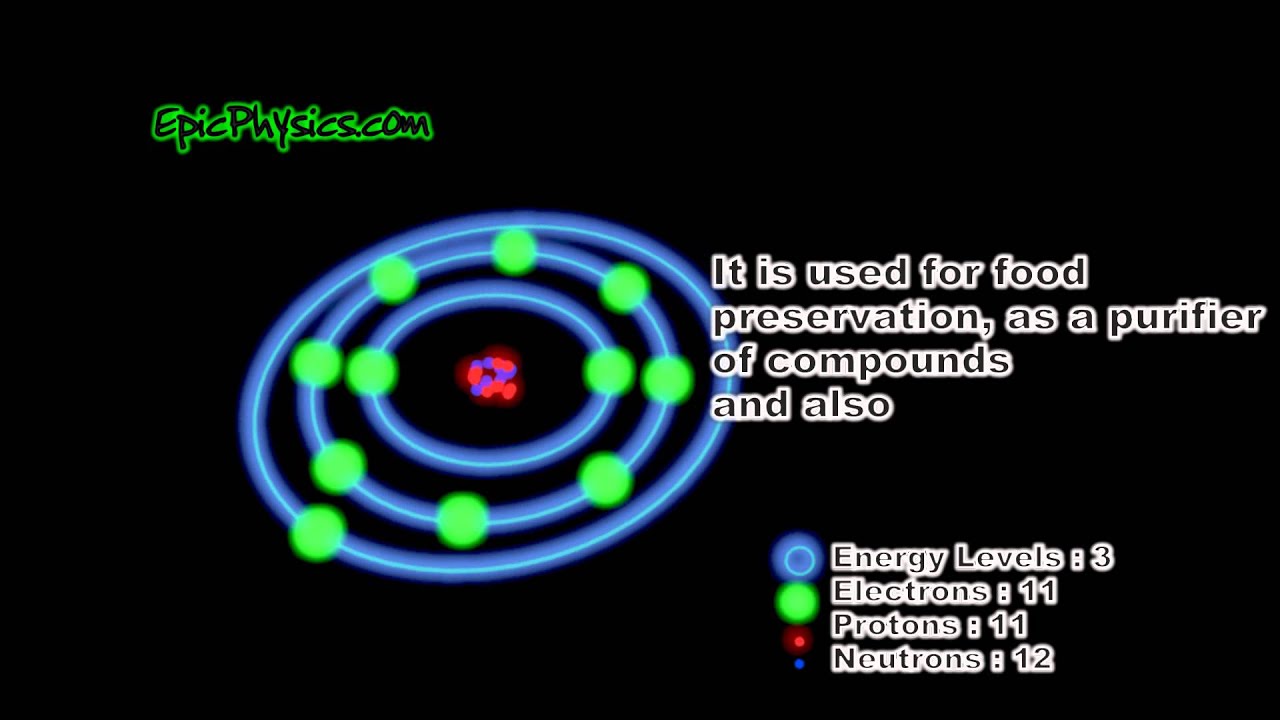
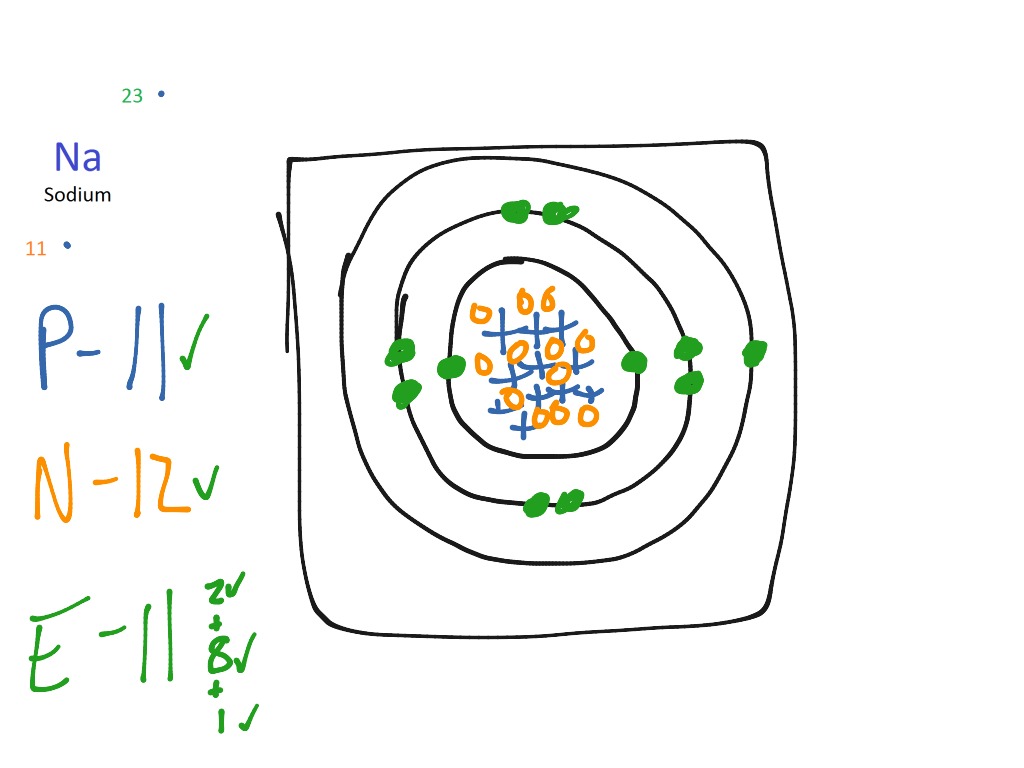
Sodium bohr diagram. Medicine agriculture obtained from. Neither of them has an outer shell that is filled so these atoms are not very stable on their own. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. If it is in an ionic bond i believe sodium has 10 electrons outer valence shells and chlorine has 18 electrons.
Both elements have three electron shells. One for each proton. A bohr model is constructed by drawing a circle in the middle. The same with the second ring.
Contains lots of information about sodiums most famous compound. In the bohr model electrons are pictured as traveling in circles at different shells depending on which element you have. A bohr diagram is a simplified visual representation of an atom that was developed by danish physicist niels bohr in 1913. The external links below are not a part of this site and their content is not the responsibility of this site.
On that ring put 2 dots. Bohr diagrams bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In atomic physics the rutherfordbohr model or bohr model or bohr diagram introduced by niels bohr and ernest rutherford in 1913 depicts the atom as a small positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleussimilar to the structure of the solar system. From the latin word natrium sodium uses.
Now look carefully at the following bohr models of sodium and chlorine. Then a ring around it. Bohr diagram for sodium and chlorine.