Crf Case Report Form Templates
These templates are for use with phase i and ii dcp chemoprevention trials.
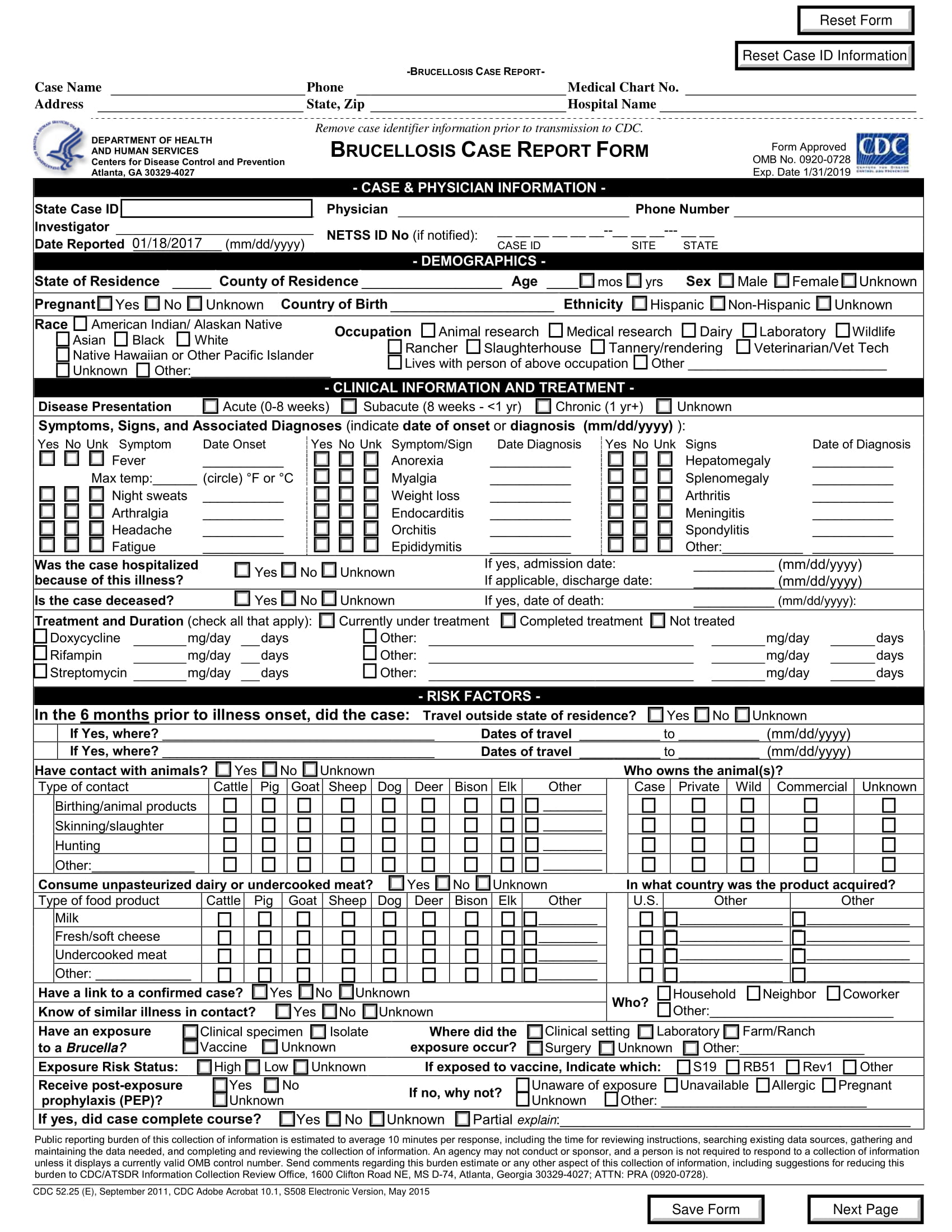
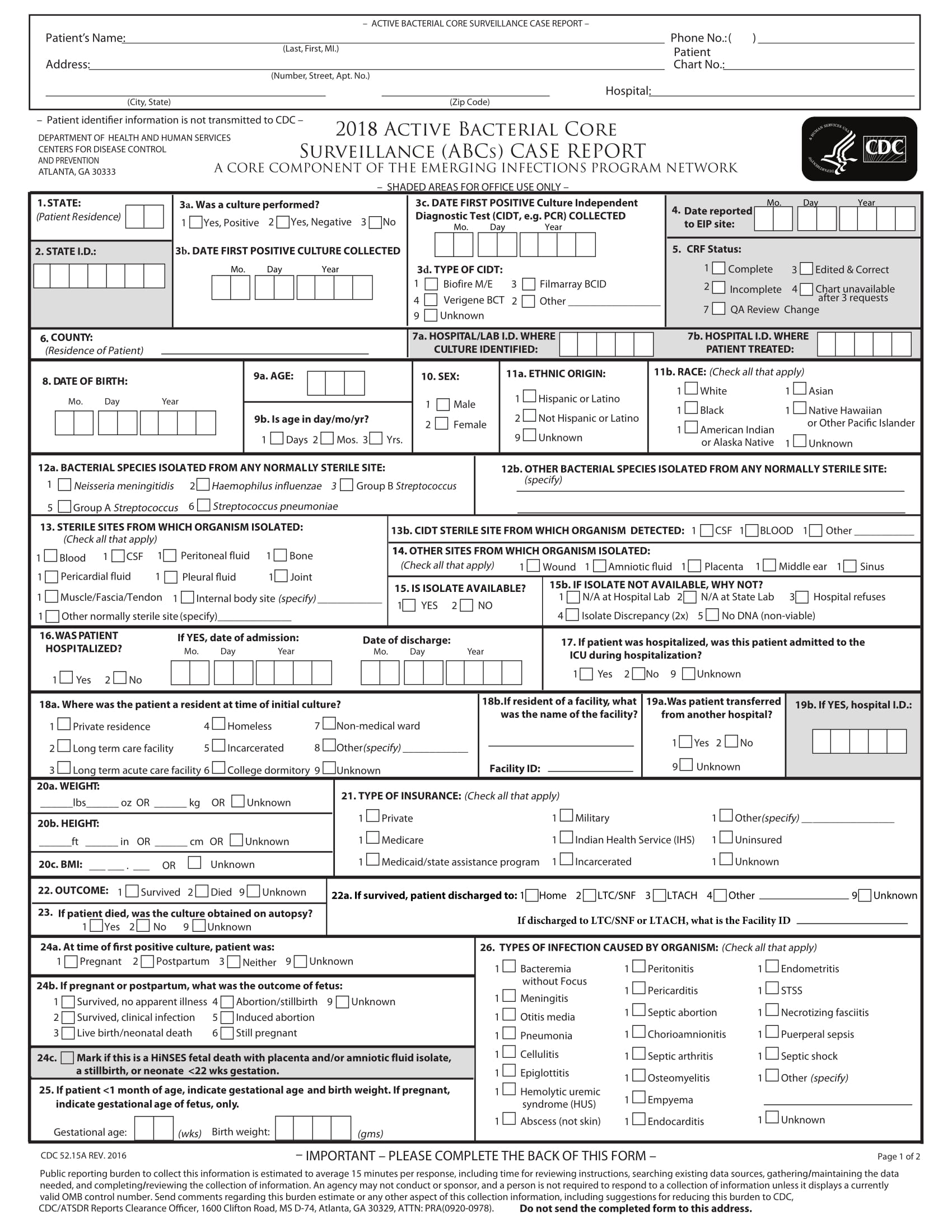
Crf case report form templates. Case report form completion guidelines case report form design electronic case report form standard templates introduction. Case report form crf is a specialized document in clinical research. For best results clear form between searches. Clinical trials and research governance have a crf template which can be adapted to suit the study requirements.
The cdash standards identify those elements that should be captured on a case report form crf. Staff with sample case report forms crf templates. Its development represents a significant part of the clinical trial and can affect study success. The development of an electronic case report form ecrf the electronic case report from ecrf played a pioneering role in the digitalization and introduction of ever new technologies into clinical research and enjoys great popularity.
Sops the respective templates and the responsibilities for data management procedures within a study. D d m m y y visit 1 screening inclusion criteria date of assessment d d m m y y inclusion criteria yes no na 1. Case report form template appendix 1 to sop s 1039 v2 nov 2016 subject id. These templates are consistent with the fdas cdash clinical data acquisition standards harmonization standards.
Case report form crfsource document templates were created for university of wisconsin madison researchers. Users are able to search the library to find crf modules and guidelines of interest. A case report form crf is designed to collect the patient data in a clinical trial. From the manage case report forms page download the crf you will use as a basis for defining the crf.
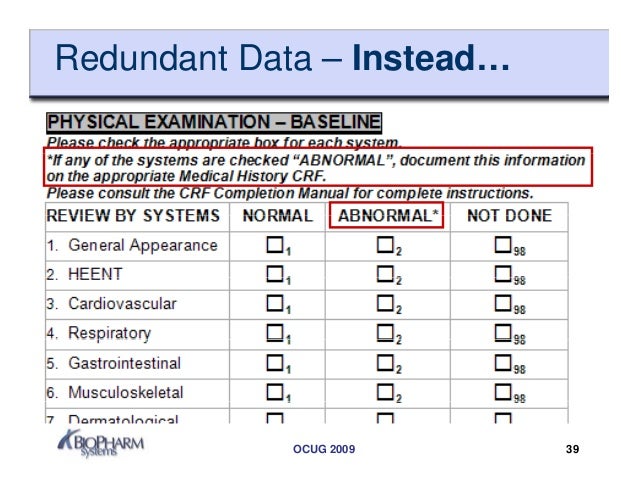
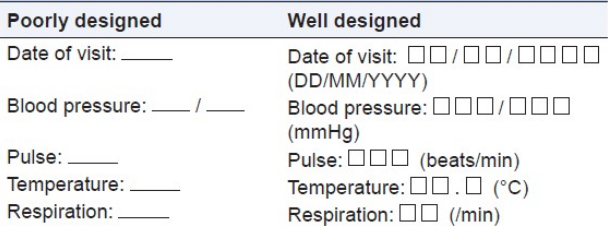
As indicated in the contract a complete set of study specific case report forms shall. The way the crf is designed depends on the type of variables that are collected. General instructions for completion of the case report forms crf completion of crfs a crf must be completed for each study participant who is successfully enrolled received at least one dose of study drug for reasons of confidentiality the name and initials of the study participant should. Design and development of case report forms template.
Note that when you save the excel file in this process the excel filename the name that precedes xls is not used by openclinica in any way. Case report form template. The templates contain recommended content and format and may be downloaded and modified with study specific information for each trial. The crf library aka library of case report form modules and guidelines contains the ninds crf modules ie form templates and various guideline documents that have been created through the ninds cde project.
It should be study protocol driven robust in content and have material to collect the study specific data.