Device Master Record Template

The failure to maintain the device master record dmr or in some cases the complete failure to create one.
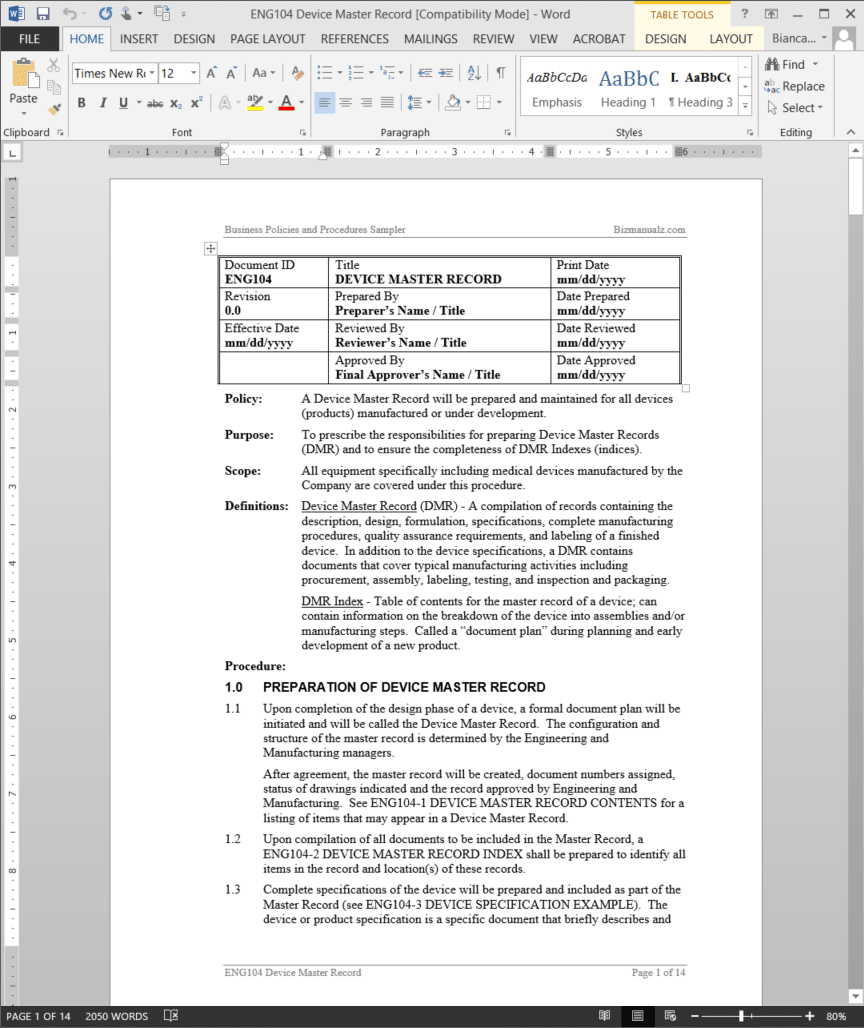
Device master record template. It is further discussed in 21 cfr 8203 g design output. The device master record contents template is a listing of items that may appear in a device master record. These requirements are in the qs regulation because the device master record is the. Dmr device master record.
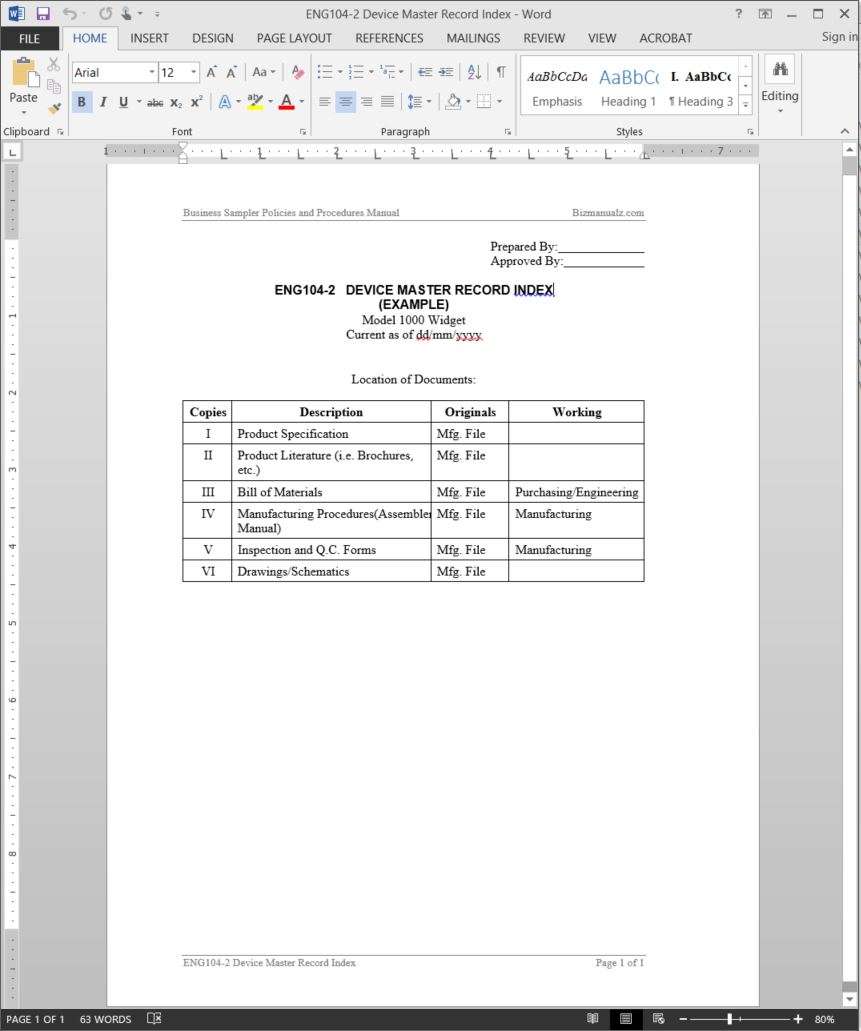
Section 8203j of the federal code defines device master record. Upon compilation of all documents to be included in the master record a device master record index template should be prepared to identify all items in the record and locations of these records. D is going to discuss a terrible trend he is starting to see among device manufacturers. The configuration and structure of the master record is determined by the engineering and manufacturing.
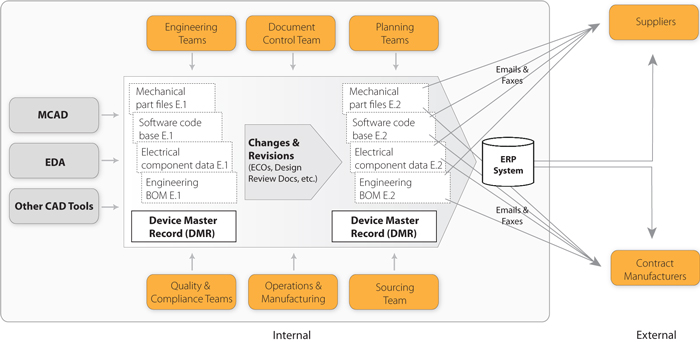
The finished design output is the basis for the device master record. The total finished design output consists of the device its packaging and labeling and. Device master record dmr means a compilation of records containing the procedures and specifications for a finished device. Each manufacturer shall ensure that each dmr is prepared and approved in accordance with 82040.
The dmr for each type of device shall include or refer to the location of the following information. 820181 device master record. Eng104 2 device master record index includes descriptions such as product specification and product literature. This package includes one exampletemplate dmr and one dhf.
Upon completion of the design phase of a device a formal document plan will be initiated and will be called the device master record. Ladies and gentlemen in this weeks guidance dr. This procedure describes the requirements for device master records dmrs and design history files dhfs. Dhr device.
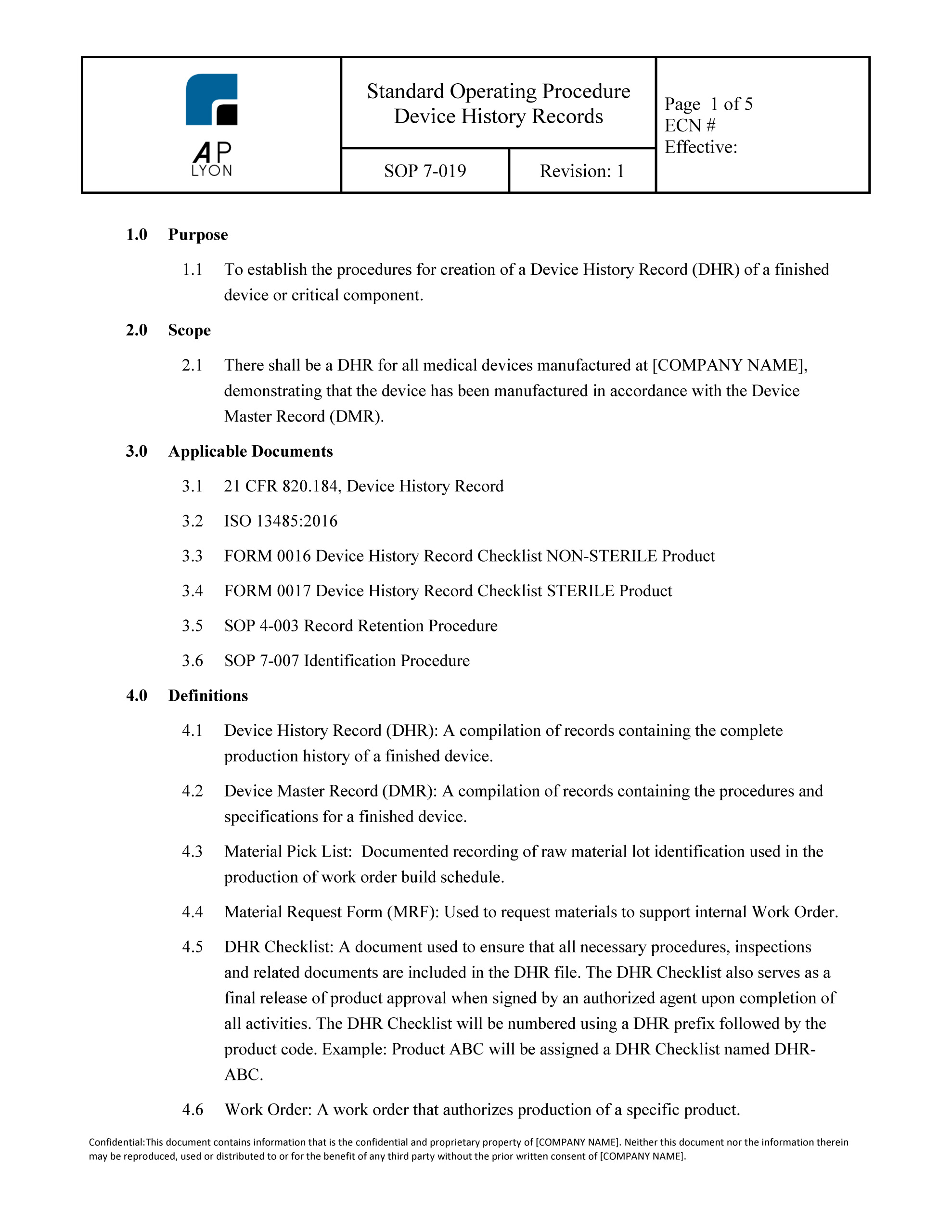
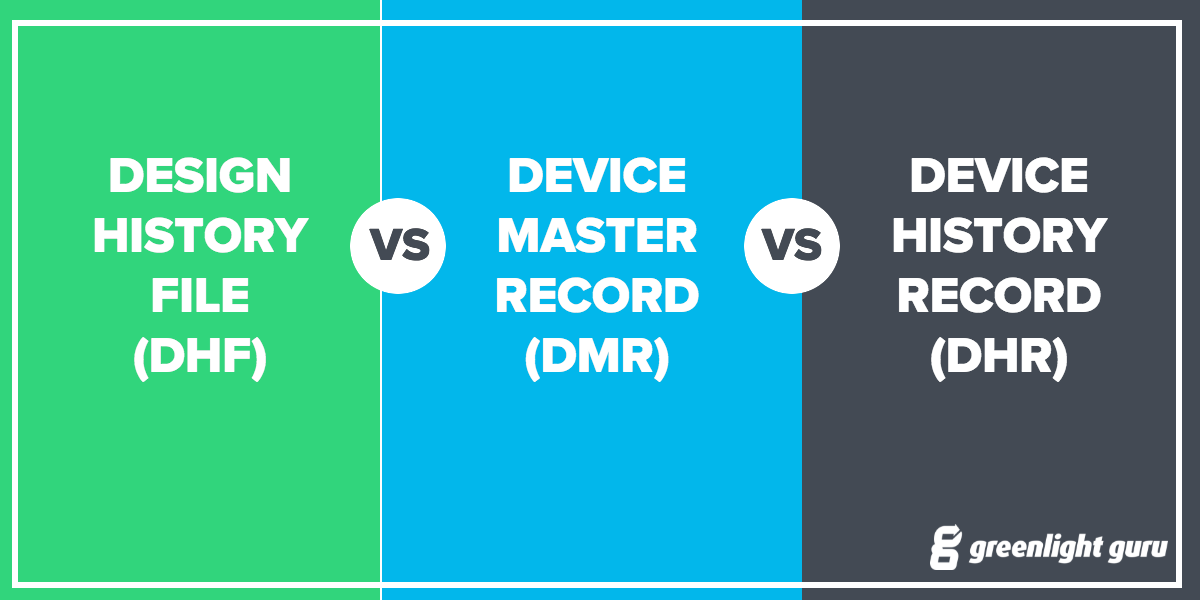
Dhf design history file. Much like the dhf is the history of the design the dhr is the history of the device. While these three acronyms can see confusing and easily interchangeable when you first hear them if you look at the actual terms theyre surprisingly descriptive. The food and drug administration fda requires manufacturers of medical devices to create and maintain a device master record dmr.
Each manufacturer shall maintain device master records dmrs. This procedure applies to in vitro diagnostic ivd products. Device master records should be technically correct contain andor reflect the approved device and process designs be under change control contain the release or other control date contain an approval signature and be directed toward the intended user. Device master record index template.